NASA Scientists Cook Up Building Blocks of Life in Lab
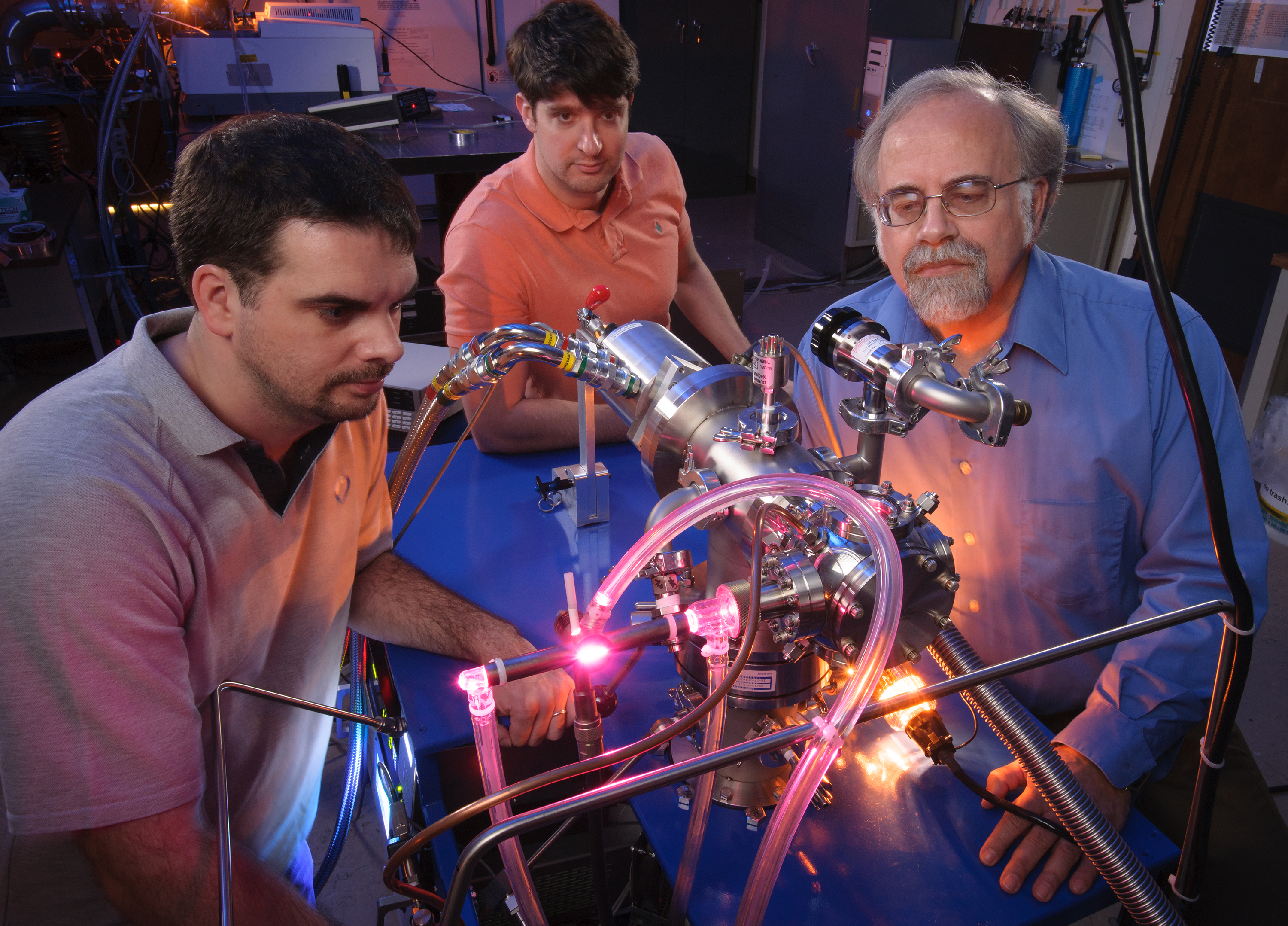
Many of the chemical ingredients necessary for life as we know it were available on the early Earth, and should be present on exoplanets as well, new research suggests.
Researchers at NASA's Ames Research Center in California generated three key components of RNA (ribonucleic acid) and DNA (deoxyribonucleic acid) in the lab, by exposing commonly occurring ring-shaped molecules of carbon and nitrogen to radiation under spacelike conditions.
"Nobody really understands how life got started on Earth," Scott Sandford, a space science researcher at Ames, said in a statement. "Our experiments suggest that once the Earth formed, many of the building blocks of life were likely present from the beginning. Since we are simulating universal astrophysical conditions, the same is likely wherever planets are formed." [7 Theories on the Origin of Life]
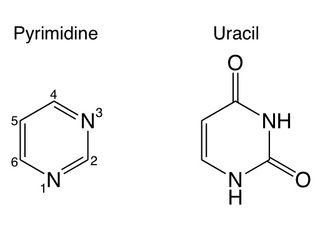
Sandford and his colleagues worked with pyrimidine, a ring-shaped molecule often found in meteorites. The rings hold carbon atoms, but the presence of nitrogen makes pyrimidine less stable than other carbon-rich compounds, researchers said. As a result, pyrimidine is easily destroyed by radiation, which is prevalent in interstellar space.
"We wanted to test whether pyrimidine can survive in space, and whether it can undergo reactions that turn it into a more complicated organic species," Sandford said in the same statement.
Pyrimidine should be vulnerable to destruction when traveling through the universe as a gas. But the researchers reasoned that some molecules might be able to survive if they find their way into interstellar clouds of dust and gas.
Such clouds could serve as a shield, absorbing much of the radiation on the outer edges and keeping it from reaching the interior. Safe inside the clouds, the pyrimidine molecules would freeze onto dust grains, which might allow them to survive any radiation to which they would later be exposed.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
To test their idea, the scientists exposed an ice sample containing pyrimidine to ultraviolet radiation in a vacuum at temperatures as low as minus 440 degrees Fahrenheit (minus 262 degrees Celsius) —conditions similar to those experienced in interstellar space.
When frozen in ice consisting mainly of water, but also containing ammonia, methanol or methane, the pyrimidine was much less vulnerable to radiation than it would be as a free-floating gas. Instead of destroying the molecules, the radiation transformed it into new species, including uracil, cytosine and thymine — three of the "nucleobases" that make up DNA and RNA.
"We are trying to address the mechanisms in space that are forming these molecules," Ames researcher Christopher Materese said. "Considering what we produced in the laboratory, the chemistry of ice exposed to ultraviolet radiation may be an important linking step between what goes on in space and what fell to Earth early in its development."
Although scientists know that pyrimidine is found in meteorites, they are still uncertain about its ultimate origins. Like the more stable, carbon-rich polycyclic aromatic hydrocarbons (PAHs), considered as potential material to kick-start life, pyrimidine may be produced by the dying breaths of red-giant stars or in clouds of interstellar gas and dust, researchers said.
Follow us @Spacedotcom, Facebook or Google+. Originally published on Space.com.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

Nola Taylor Tillman is a contributing writer for Space.com. She loves all things space and astronomy-related, and enjoys the opportunity to learn more. She has a Bachelor’s degree in English and Astrophysics from Agnes Scott college and served as an intern at Sky & Telescope magazine. In her free time, she homeschools her four children. Follow her on Twitter at @NolaTRedd