'Impossible' Cloud On Saturn's Moon Titan May Resemble Earth's Ozone Killers
A strange cloud on Titan that seems to appear out of nowhere may form through process similar to one that depletes the ozone layer at Earth's poles. This process involves reactions with solid crystals rather than vapor.
The weird cloud, which NASA officials described as "impossible" in a statement, is made of dicyanoacetylene (C4N2), which is one of several hydrocarbons that give Titan's atmosphere it's orange-brown hue. One cloud just like it was first seen by Voyager 1, which passed by Saturn in 1980. Strangely, though, there didn't seem to be enough dicyanoacetylene in Titan's atmosphere to make such a cloud — only 1 percent of the amount needed.
Observations of this newer cloud, taken by the Cassini orbiter currently exploring Saturn's system, yielded similar results: The spacecraft saw a high-altitude cloud, but there was not enough raw material to make one. At first, the scientists thought the problem was that the Voyager instrument wasn't sensitive enough to pick up the dicyanoacetylene. But when Cassini got the same result, that explanation was scrapped, researchers said in the NASA statement.
Clouds are usually formed via condensation; a gas will rise to a certain height before cooling enough to turn to liquid vapor. This is what water does on Earth, forming clouds, and what methane does on Titan.
In the upper atmosphere, the process differs slightly; in that case, warm air is pushed to the poles and then sinks, where it condenses into clouds.
Carrie Anderson, a planetary scientist at NASA's Goddard Space Flight Center in Greenbelt, Maryland, led a team at Goddard and the California Institute of Technology that proposed a different model for those clouds' formation: reactions among solid ice particles.
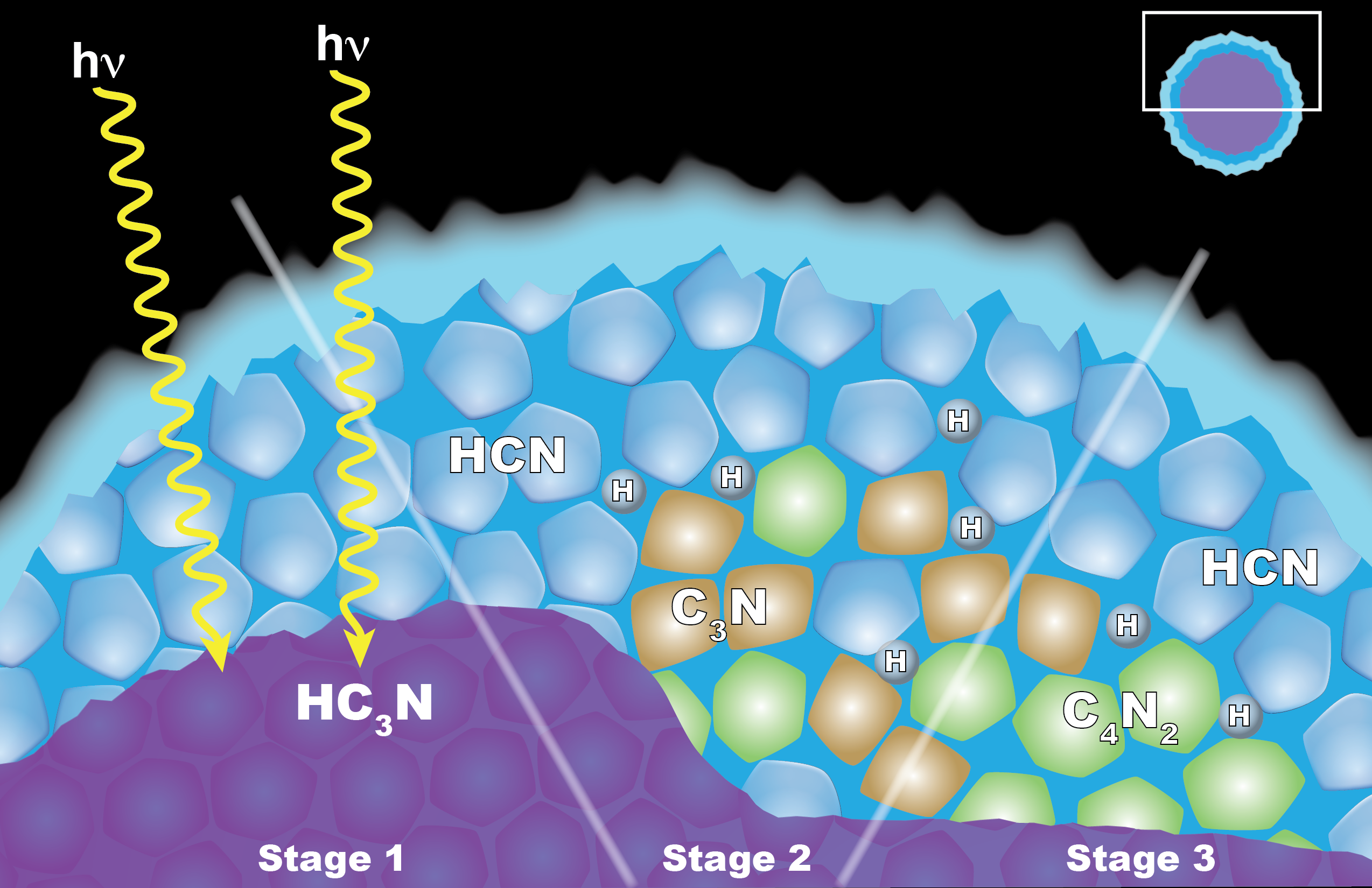
Under this process, crystals of another hydrocarbon, cyanoacetylene (HC3N), would condense as the gas moves downward through Titan's upper atmosphere. On the way, the crystals get coated by hydrogen cyanide (HCN). The little coated particles get hit with ultraviolet light from the sun, and the result is a reaction that forms dicyanoacetylene ice and hydrogen.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
This process is similar to one on Earth involving clouds that sometimes form in the stratosphere and chlorine-bearing chemical pollutants. The chlorine chemicals stick to the ice crystals, react when hit with UV light and create ozone-destroying chemicals.
The study appears in the journal Geophysical Research Letters.
You can follow Space.com on Twitter @Spacedotcom. We're also on Facebook & Google+. Original story on Space.com.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

Jesse Emspak is a freelance journalist who has contributed to several publications, including Space.com, Scientific American, New Scientist, Smithsonian.com and Undark. He focuses on physics and cool technologies but has been known to write about the odder stories of human health and science as it relates to culture. Jesse has a Master of Arts from the University of California, Berkeley School of Journalism, and a Bachelor of Arts from the University of Rochester. Jesse spent years covering finance and cut his teeth at local newspapers, working local politics and police beats. Jesse likes to stay active and holds a fourth degree black belt in Karate, which just means he now knows how much he has to learn and the importance of good teaching.









