Buried in the Cat's Paw Nebula lies one of the largest space molecules ever seen
The molecule 2-methoxyethanol could reveal how the cosmos grew so complex.
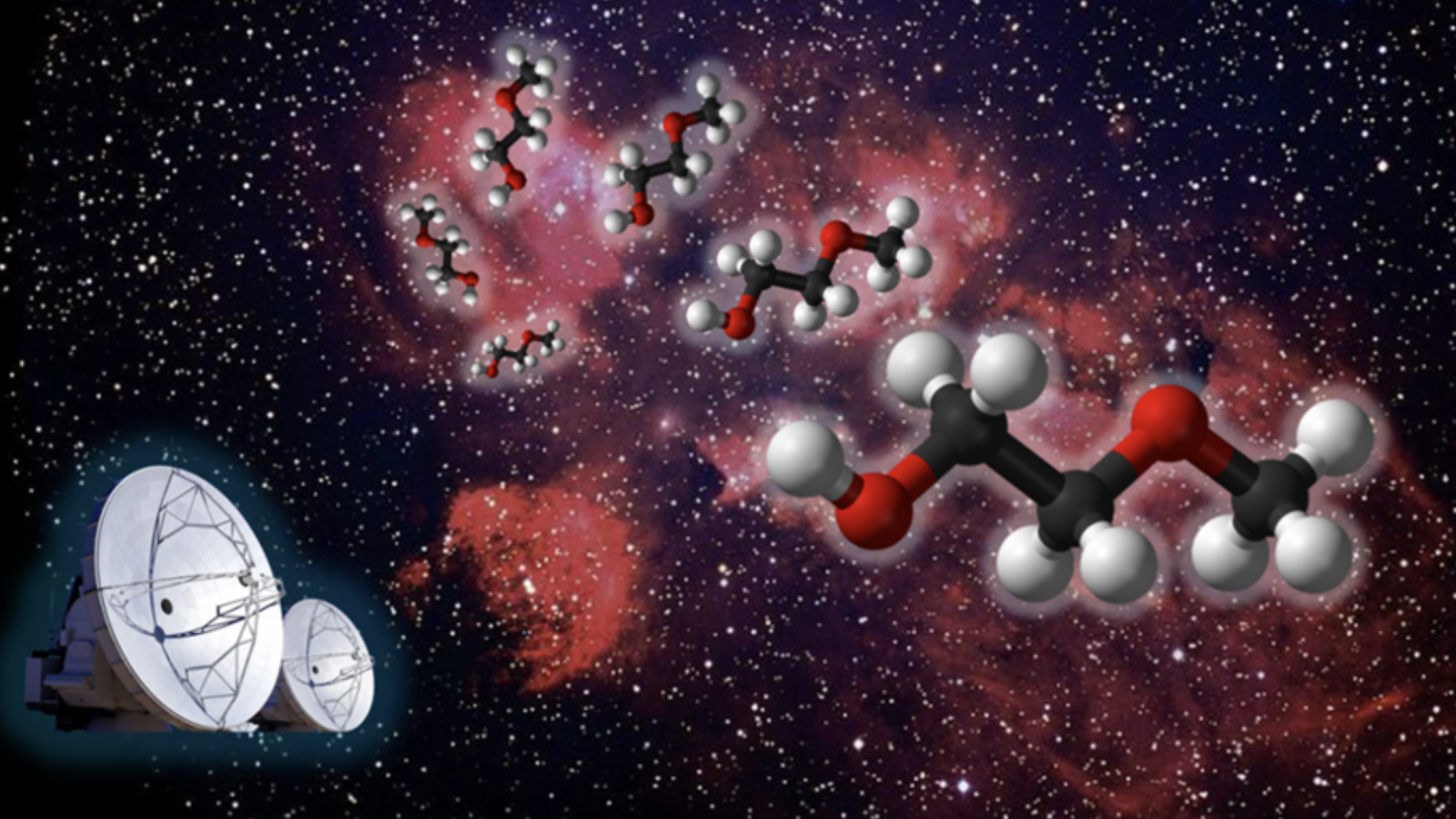
Scientists have discovered a hitherto unknown space molecule while investigating a relatively nearby region of intense star birth, a cosmic spot about 5,550 light-years away. It's part of the Cat's Paw Nebula, also known as NGC 6334.
The team, led by Zachary Fried, a graduate student at the Massachusetts Institute of Technology (MIT), examined a section of the nebula known as NGC 6334I with the Atacama Large Millimeter/submillimeter Array (ALMA). This revealed the presence of a complex molecule known as 2-methoxyethanol, which had never been seen before in the natural world, though its properties had been simulated in labs on Earth.
Discovering molecule 2-methoxyethanol was remarkable. It contains 13 atoms, which may not sound like a lot, but only six molecules have been discovered in space with an atom count beyond this. This molecule also represents the largest and most complex "methoxy" molecule found in space to date, referring to a chemical with a methyl group atom bound to an oxygen atom.
Related: Scientists find record-breaking collection of molecules in 2 extremely ancient galaxies
"Our group tries to understand what molecules are present in regions of space where stars and solar systems will eventually take shape," Fried said. "This allows us to piece together how chemistry evolves alongside the process of star and planet formation."
Interestingly, the same team also hunted for 2-methoxyethanol in another region of space called IRAS 16293-2422B, home to four newborn protostars located in the star-forming region of Rho Ophiuchi that sits around 359 light-years away from us. This could hint at more diversity in the chemical composition of star-forming regions.
ALMA knew what to look for in the Cat's Paw
Fried and colleagues didn't go into the investigation of NGC 6334I and IRAS 16293-2422B without any foundation. They already had a good idea of the molecule they would be hunting for with ALMA, an array of 66 radio telescopes located in the Atacama desert in Northern Chile. Basically, they received a tip from machine learning models that suggested they hunt for 2-methoxyethanol.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
The group then measured and analyzed the rotational spectrum of 2-methoxyethanol on Earth, which Fried described as "the unique patterns of light they give off as they tumble end-over-end in space."
"These patterns are fingerprints or barcodes for molecules," the MIT researcher added. "To detect new molecules in space, we first must have an idea of what molecule we want to look for, then we can record its spectrum in the lab here on Earth, and then finally we look for that spectrum in space using telescopes.
"The barcode matched!"

"Ultimately, we observed 25 rotational lines of 2-methoxyethanol that lined up with the molecular signal observed toward NGC 6334I, thus resulting in a secure detection of 2-methoxyethanol in this source," Fried said.
This successful detection then allowed the team to derive physical parameters of the molecule in conjunction with NGC 6334I, including the abundances at which it exists and the molecule's excitation temperature.
"It also enabled an investigation of the possible chemical formation pathways from known interstellar precursors," Fried added.
Discoveries such as this allow scientists to better understand how increasingly complex molecules emerge during the formation of stars, as well as when planets begin coming together around those stars.
"Continued observations of large molecules and subsequent derivations of their abundances allow us to advance our knowledge of how efficiently large molecules can form and by which specific reactions they may be produced," Fried concluded. "Additionally, since we detected this molecule in NGC 6334I but not in IRAS 16293-2422B, we were presented with a unique opportunity to look into how the differing physical conditions of these two sources may be affecting the chemistry that can occur."
The team's research was published on April 12 in the journal The Astrophysical Journal Letters.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

Robert Lea is a science journalist in the U.K. whose articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space, Newsweek and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University. Follow him on Twitter @sciencef1rst.
-
Unclear Engineer With all sorts of elements floating around in space in extremely low concentrations, constantly being bombarded with ionizing radiation, I am not surprised that a lot of different chemicals can be created.Reply
But, what happens when all of those chemicals condense into a planet? I would expect some to destroy others, and some to build even more complex compounds.
So, I am wondering if these researchers have any ideas about whether 2-methoxyethanol has any relevance to biological development on a planet - particularly one that starts with an atmosphere low on oxygen and high on CO2.