'Wonder material' found in lunar samples hints at moon's origins
"This finding may reinvent the understanding of chemical components, geography episodes and the history of the moon."
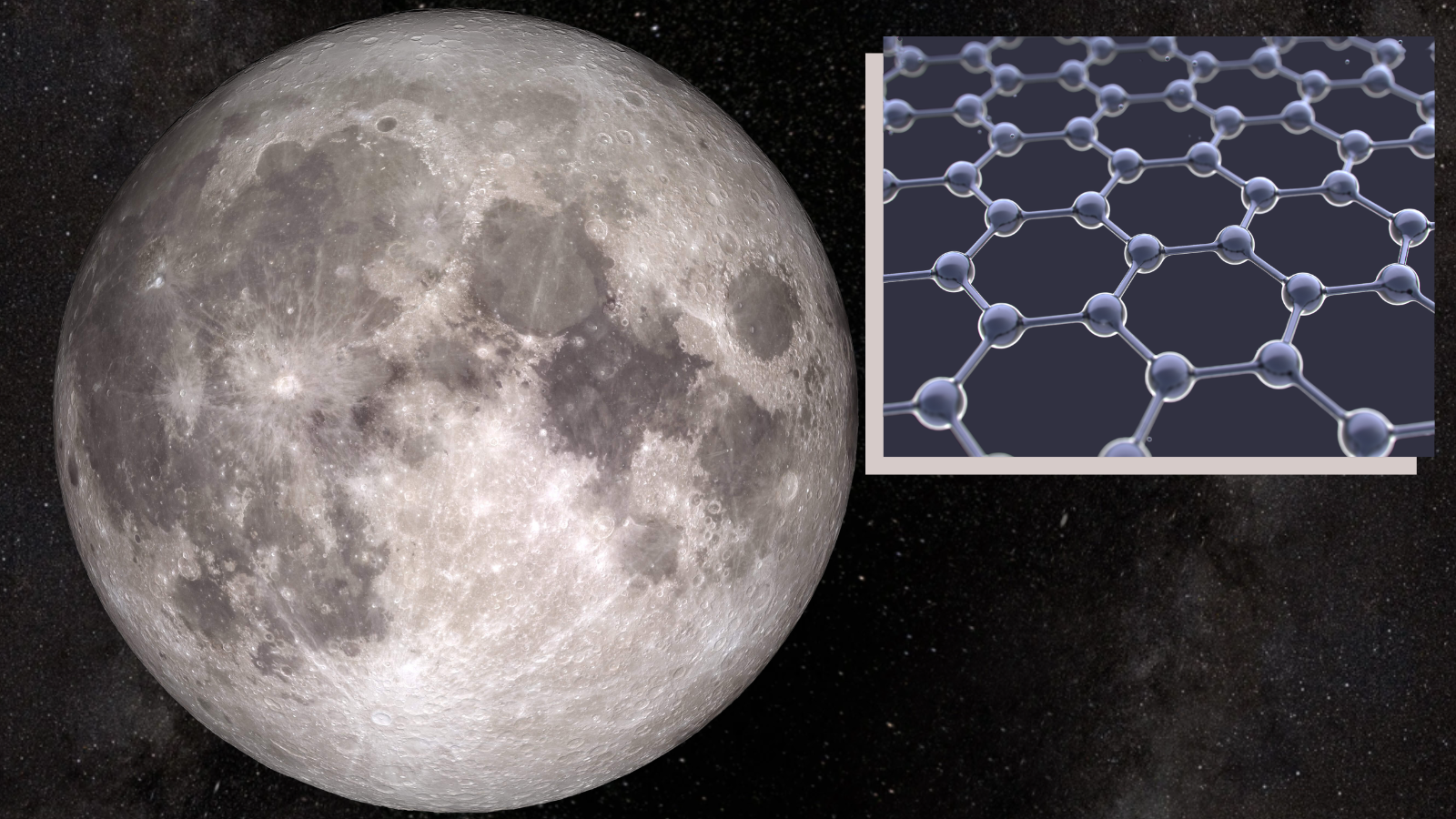
Scientists have discovered naturally formed graphene flakes in lunar soil samples, potentially shedding light on the mystery surrounding the moon's formation.
The samples were collected from the moon in 2020 by Chang'e-5 (CE-5), the fifth lunar exploration mission of the Chinese Lunar Exploration Program and China's first mission to return samples to Earth.
Graphene is a form of carbon, or "allotrope," made up of a single layer of atoms arranged in a honeycomb structure. It was first discovered in 2004 by Andre Geim and Konstantin Novoselov. In 2010, the duo received the Nobel Prize in Physics for the discovery because of graphene's remarkable physical properties and its potential applications in electronics, energy storage, sensing and biomedicine, among many others.
"Graphene has revolutionized the research of condensed matter physics and materials science with its novel physical phenomena and extraordinary properties," the lunar sample team wrote in a recent paper published in the journal National Science Review.
But why were scientists looking for graphene on the moon to begin with?
Could graphene reveal how the moon formed?
The moon's origins are still up for debate, though many theories have been put forward throughout the years. One theory in particular, the giant impact hypothesis, has gained popularity.
This hypothesis proposes that Earth collided with a Mars-size planet around 4.5 billion years ago, producing debris in Earth's orbit that eventually formed the moon. Giant impacts like this were common in the inner solar system when Earth was coming together in the turbulent early era of our planetary system.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
Related: The moon's thin atmosphere is made by constant meteorite bombardment
While the moon's composition is similar to Earth's, samples brought back by NASA's Apollo missions revealed that our planet's long-serving lunar companion is relatively depleted in volatile elements (elements that are easily vaporized), including carbon, when compared to our planet.

Scientists postulated that the intense heat generated by such a massive collision would have caused volatile elements to vaporize and escape, leaving behind a carbon-depleted body. The Apollo samples also showed a similarity in isotope composition between Earth and the moon. Isotopes are atoms of the same element with different numbers of neutrons in their nuclei, so these results further support the idea that the moon formed from material flung away from Earth after a giant impact.
This new data from CE-5, along with recent observations that have detected carbon ion fluxes emanating from the moon, indicate there might be native or indigenous carbon present there. That challenges the existing consensus around this theory.
"It is highly desirable to unravel the crystalline structure of the [moon's] indigenous carbon," wrote the team. This is because carbon is a fundamental element for understanding the formation and evolution of planetary bodies, with its shape and structure determined by the process of its formation.
"Since graphene has been routinely prepared by using artificial techniques with distinct morphologies and properties as determined by the specific formation process, the composition and structure characterization of natural graphene would provide rich information on the geologic evolution of parent bodies," the team said.

The team behind the discovery of graphene in lunar samples analyzed an "olive-shaped" lunar soil sample 2.9 millimeters long and 1.6 mm wide.
To search for graphene carbon within this sample, the researchers used a variety of characterization techniques commonly used by chemists, including scanning electron microscopy (SEM) and Raman spectroscopy.
Raman spectroscopy is a light-scattering technique that allows scientists to study the vibrational, rotational and low-frequency modes of bonds within molecules. Thus, this technique provides insights into the structure and composition of these molecules. On the other hand, SEM creates high-resolution images of the surface of a material using a focused beam of electrons. SEM can be used to determine elemental composition.

With these tools, the scientists identified embedded flakes of graphene in carbon-rich areas of the sample, ranging from two to seven layers thick. The team also observed that the layered graphene forms a shell structure enclosing a core of complex compounds.
This suggests a bottom-up synthesis process rather than creation by exfoliation, the separation of layers by breaking bonds between them, which typically involves a high-temperature reaction.
In addition to finding graphene in the CE-5 returned lunar soil samples, the scientists found an iron-containing compound present only in areas of the sample where carbon was found.
This is intriguing because iron-containing minerals on the moon, such as olivine and pyroxene, may have played a role in catalyzing carbon's transformation into graphene.
Volcanic activity, solar winds, or meteorite impacts could have provided the necessary energy for this transformation, which may have created the high-pressure, high-temperature environments necessary for carbon to convert into graphene.
"This finding may reinvent the understanding of chemical components, geography episodes, and the history of the moon," the team behind this discovery said.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

A chemist turned science writer, Victoria Corless completed her Ph.D. in organic synthesis at the University of Toronto and, ever the cliché, realized lab work was not something she wanted to do for the rest of her days. After dabbling in science writing and a brief stint as a medical writer, Victoria joined Wiley’s Advanced Science News where she works as an editor and writer. On the side, she freelances for various outlets, including Research2Reality and Chemistry World.