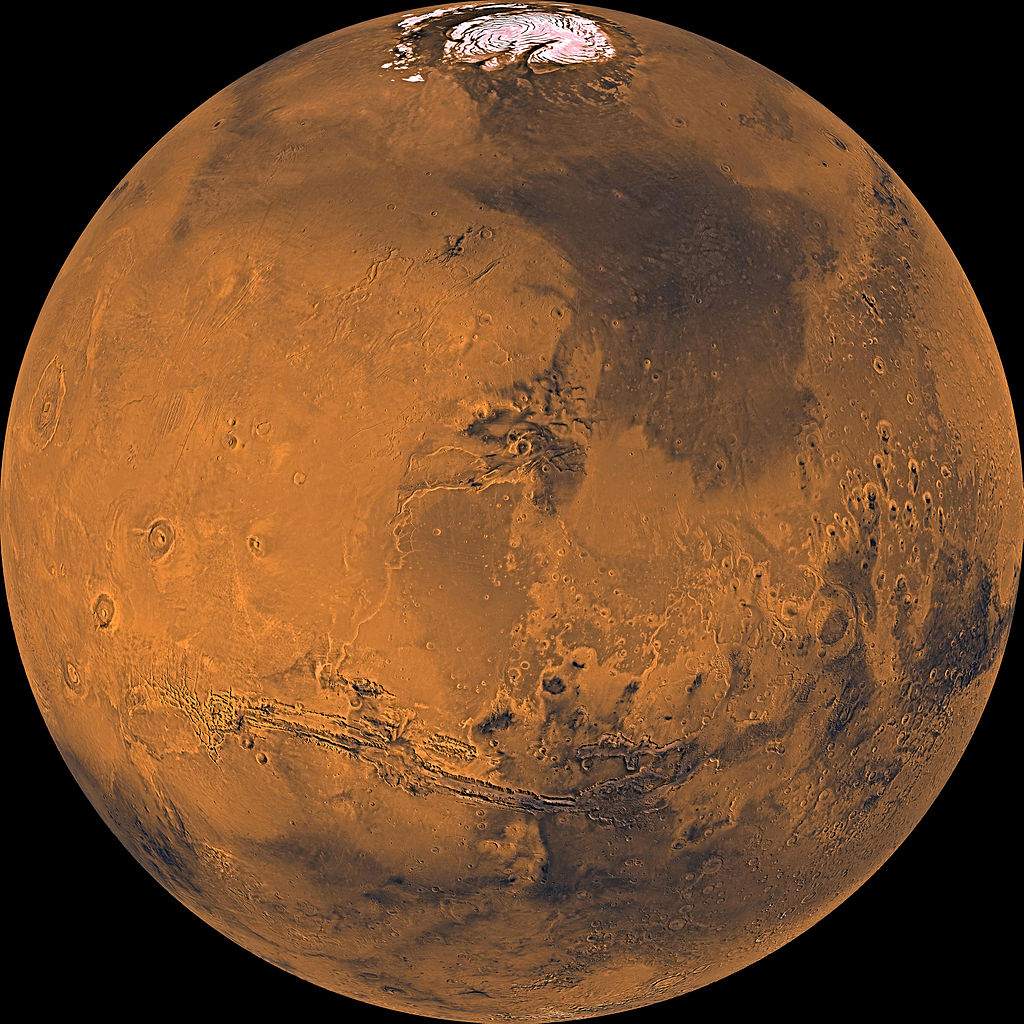
On Mars, deep-water diversity has stood the test of time, meteorites show
Breaking space news, the latest updates on rocket launches, skywatching events and more!
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered daily
Daily Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!

Twice a month
Strange New Words
Space.com's Sci-Fi Reader's Club. Read a sci-fi short story every month and join a virtual community of fellow science fiction fans!
The water buried deep within Mars likely came from at least two very different sources long ago, a new study suggests.
"These two different sources of water in Mars' interior might be telling us something about the kinds of objects that were available to coalesce into the inner, rocky planets," Jessica Barnes, an assistant professor of planetary sciences in the University of Arizona Lunar and Planetary Laboratory, said in a statement.
"This context is also important for understanding the past habitability and astrobiology of Mars," Barnes added.
Related: What is Mars made of?
Barnes and her colleagues analyzed two Mars meteorites: Northwest Africa (NWA) 7034, also known as Black Beauty, and Allan Hills 84001 (ALH84001), probably the most famous Red Planet rock of all time. In the mid-1990s, a team of researchers announced that they'd found compelling evidence of Martian life in ALH84001. Most other scientists were not convinced, and the claim remains controversial, and debated, to this day.
Barnes and her team determined the hydrogen-isotope compositions of Black Beauty and ALH84001, which interacted with water in the Martian crust about 1.5 billion years ago and 3.9 billion years ago, respectively.
Isotopes are versions of an element that have different numbers of neutrons in their atomic nuclei. For example, the hydrogen in "normal" water has no neutrons in its nucleus, whereas the hydrogen in deuterium, or "heavy water," has one.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
Studies of Mars meteorites over the years have found a wide variety of hydrogen-isotope ratios. But, in the new study, which was published online Monday (March 30) in the journal Nature Geoscience, Barnes and her team found that Black Beauty and ALH84001 have very similar amounts of normal versus heavy hydrogen.
That ratio is roughly the same as that observed for much younger rocks, suggesting that not much has changed, hydrogen-isotope wise, for the past 4r billion years or so on Mars. In addition, the isotope ratio is about halfway between that seen in crustal rocks here on Earth and the ratio observed in the Martian atmosphere, which has a lot more heavy hydrogen.
The Martian atmosphere is so "fractionated" because charged particles from the sun have preferentially blown the lighter normal hydrogen into space, scientists say.
"We thought, OK, this is interesting, but also kind of weird," Barnes said of the new results. "How do we explain this dichotomy where the Martian atmosphere is being fractionated, but the crust is basically staying the same over geological time?"
Researchers have long thought that Mars' mantle — the thick rock layer beneath the thin crust — sports a similar hydrogen-isotope ratio to that of Earth. So, the variability in isotope ratios seen in Mars meteorites was chalked up to terrestrial contamination or alteration by the Red Planet's atmosphere as the rocks, blasted free by powerful impacts, barreled outward toward space, Barnes said. (Crustal rocks should be similar to mantle rocks, after all; the crust is made of material from the interior that made it to the surface, cooled and solidified.)
But the presumed mantle similarities between Mars and Earth are inferred from one study of a meteorite thought to originate in the Red Planet's mantle, the researchers said. And it may be time for a rethink.
"Martian meteorites basically plot all over the place, and so trying to figure out what these samples are actually telling us about water in the mantle of Mars has historically been a challenge," Barnes said. "The fact that our data for the crust was so different prompted us to go back through the scientific literature and scrutinize the data."
This analysis revealed that two different types of Martian volcanic rocks, enriched shergottites and depleted shergottites, have different hydrogen-isotope ratios. These rocks probably represent different source material, the researchers said — different reservoirs of water in the Martian interior.
"Consequently, these features may have been inherited from the primary building blocks that constructed Mars, implying that the Martian mantle has almost always been heterogeneous because it was poorly mixed during accretion, differentiation and its subsequent thermochemical evolution," the researchers wrote in the new study.
If this is true, then, unlike Earth and the moon, Mars probably did not feature a global ocean of liquid rock shortly after its birth. A global magma ocean would have mixed everything together, erasing the distinct isotope signatures, the researchers said.
- Photo gallery: images of Martian meteorites
- Water on Mars: exploration and evidence
- Inside 3 Mars meteorites: a different path for 'building blocks of life'?
Mike Wall is the author of "Out There" (Grand Central Publishing, 2018; illustrated by Karl Tate), a book about the search for alien life. Follow him on Twitter @michaeldwall. Follow us on Twitter @Spacedotcom or Facebook.
OFFER: Save at least 56% with our latest magazine deal!
All About Space magazine takes you on an awe-inspiring journey through our solar system and beyond, from the amazing technology and spacecraft that enables humanity to venture into orbit, to the complexities of space science.

Michael Wall is a Senior Space Writer with Space.com and joined the team in 2010. He primarily covers exoplanets, spaceflight and military space, but has been known to dabble in the space art beat. His book about the search for alien life, "Out There," was published on Nov. 13, 2018. Before becoming a science writer, Michael worked as a herpetologist and wildlife biologist. He has a Ph.D. in evolutionary biology from the University of Sydney, Australia, a bachelor's degree from the University of Arizona, and a graduate certificate in science writing from the University of California, Santa Cruz. To find out what his latest project is, you can follow Michael on Twitter.


