Weird! Mercury's scorching temps may actually lead to ice.
Could Mercury's close orbit around the sun help the planet generate ice?
It sounds like a paradox, but new analysis of the planet's surface chemistry suggests that heat-generated ice may indeed be the case.
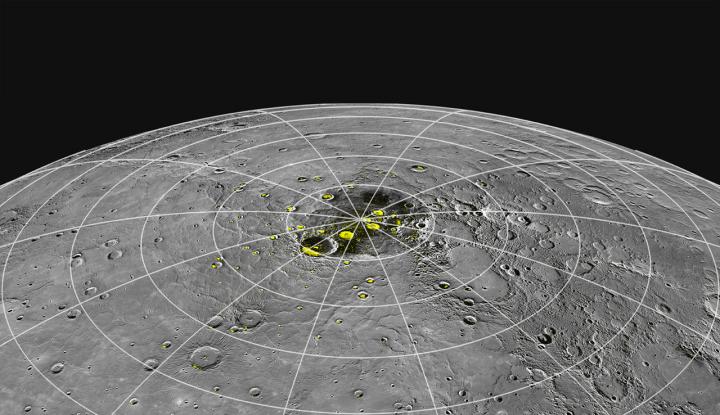
Even though daytime temperatures on Mercury soar to 750 degrees Fahrenheit (400 degrees Celsius), ice can occur in craters sheltered from the sun. There, the surface is exposed to cold space at about minus 330 F (minus 200 C).
Video: Ice on Mercury – How does it form?
Related: Photos of Mercury from NASA's Messenger spacecraft
We've known about this ice for almost a decade thanks to observations from NASA's now-defunct MESSENGER (Mercury Surface, Space Environment, Geochemistry and Ranging) spacecraft. But the explanation for how some of the ice got there, chemically speaking, remains under investigation. A new study shows how water can collect on the surface even amid these extremely hot temperatures.
"This is not some strange, out-of-left-field idea. The basic chemical mechanism has been observed dozens of times in studies since the late 1960s," Brant Jones, a researcher in Georgia Tech's School of Chemistry and Biochemistry and the lead author of the new study, said in a statement. "But that was on well-defined surfaces. Applying that chemistry to complicated surfaces like those on a planet is groundbreaking research."
The minerals on Mercury's surface contain groups of bonded oxygen and hydrogen atoms known as hydroxyls. Protons from the solar wind (the constant stream of charged particles from the sun) are common on the planet's surface, since there is not enough of a magnetic field to repel the particles.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

This study's model suggests that the magnetic field can cause protons (positively charged subatomic particles) to migrate across Mercury, so the protons can then implant themselves in the soil and the hydroxyl groups. The sun's searing heat energizes the hydroxyl groups, causing them to crash into each other. These collisions create water (which is also made from hydrogen and oxygen, just in different proportions), as well as freeing up extra hydrogen that leaves the surface and drifts above Mercury.
As for the water molecules, some of them get broken down by sunlight and dissolve into their elemental components. Other water molecules escape from the surface and fly into space. However, a few water molecules escape these fates and instead land on the poles of Mercury, making it into permanently shadowed craters.
And there, the molecules can stay, since Mercury has no substantial atmosphere that would further affect the water molecules by conducting heat, for example. While this sounds like a subtle process, over time, the water ice would add up.
The model suggests that in 3 million years, Mercury would accumulate 11 trillion tons (nearly 10 trillion metric tons) of water ice, which is roughly 10% of the observed ice on the planet. Other ice may have arrived from small worlds such as asteroids, comets and meteorites.
"It's a little like the song 'Hotel California.' The water molecules can check in to the shadows, but they can never leave," study principal investigator Thomas Orlando, who studies electron- and proton-induced surface chemistry at Georgia Tech, said in the statement.
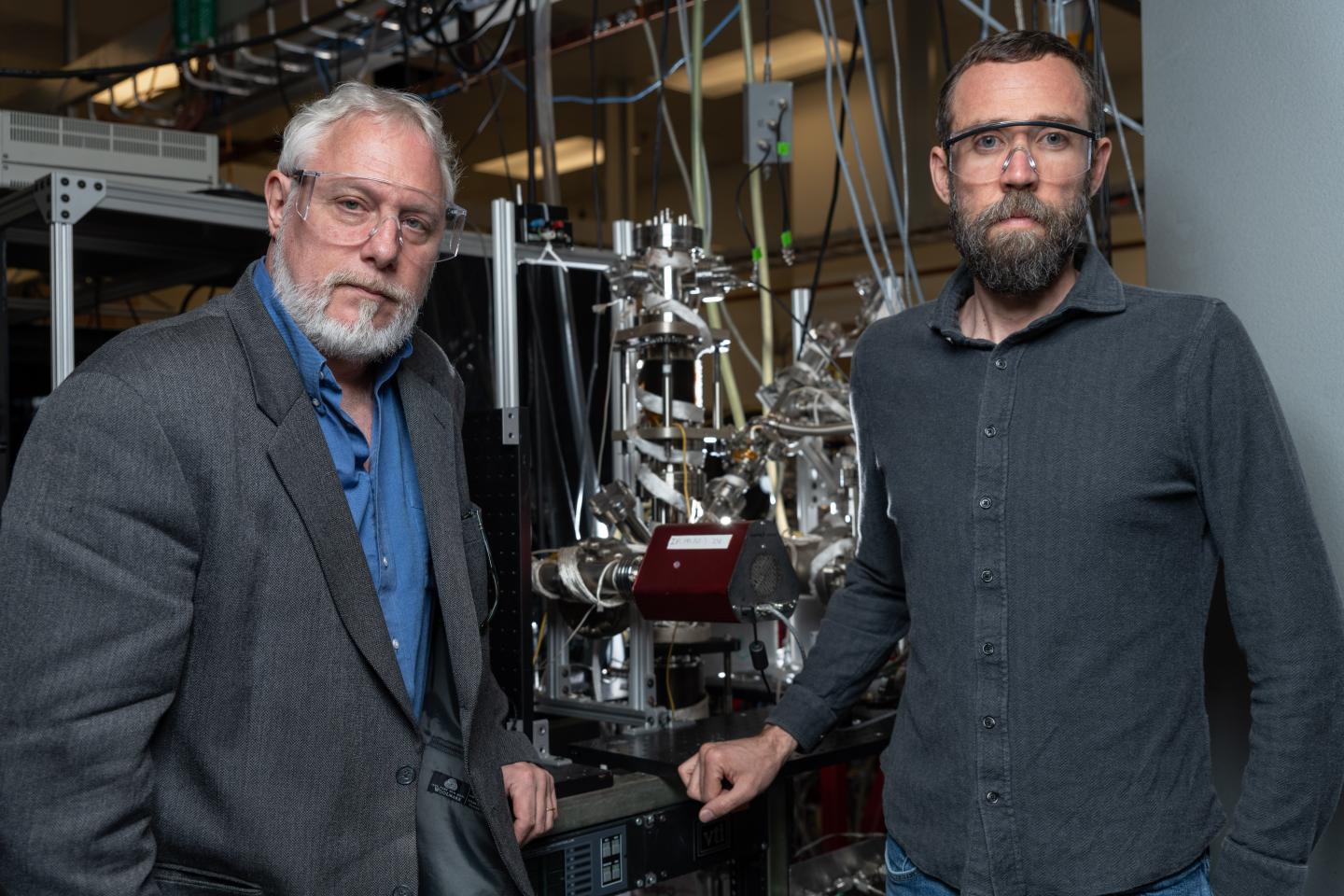
Mercury is not alone in having ice on its surface, as water ice has also been discovered on the moon and on small worlds such as asteroids and comets. These locations may have variations in water deposition, however. "The process in our model would not be anywhere near as productive on the moon. For one, there's not enough heat to significantly activate the chemistry," Jones said.
A study based on the research was published Monday (March 16) in The Astrophysical Journal Letters.
- Scorching-hot Mercury has a surprisingly icy north pole
- Water ice on Mercury: How it stays frozen (infographic)
- Shallow craters on moon and Mercury may hide thick slabs of water Ice
Follow Elizabeth Howell on Twitter @howellspace. Follow us on Twitter @Spacedotcom and on Facebook.
OFFER: Save at least 56% with our latest magazine deal!
All About Space magazine takes you on an awe-inspiring journey through our solar system and beyond, from the amazing technology and spacecraft that enables humanity to venture into orbit, to the complexities of space science.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

Elizabeth Howell (she/her), Ph.D., was a staff writer in the spaceflight channel between 2022 and 2024 specializing in Canadian space news. She was contributing writer for Space.com for 10 years from 2012 to 2024. Elizabeth's reporting includes multiple exclusives with the White House, leading world coverage about a lost-and-found space tomato on the International Space Station, witnessing five human spaceflight launches on two continents, flying parabolic, working inside a spacesuit, and participating in a simulated Mars mission. Her latest book, "Why Am I Taller?" (ECW Press, 2022) is co-written with astronaut Dave Williams.










