What makes Mars the 'Red' Planet? Scientists have some new ideas
"Mars is still the Red Planet. It’s just that our understanding of why Mars is red has been transformed."
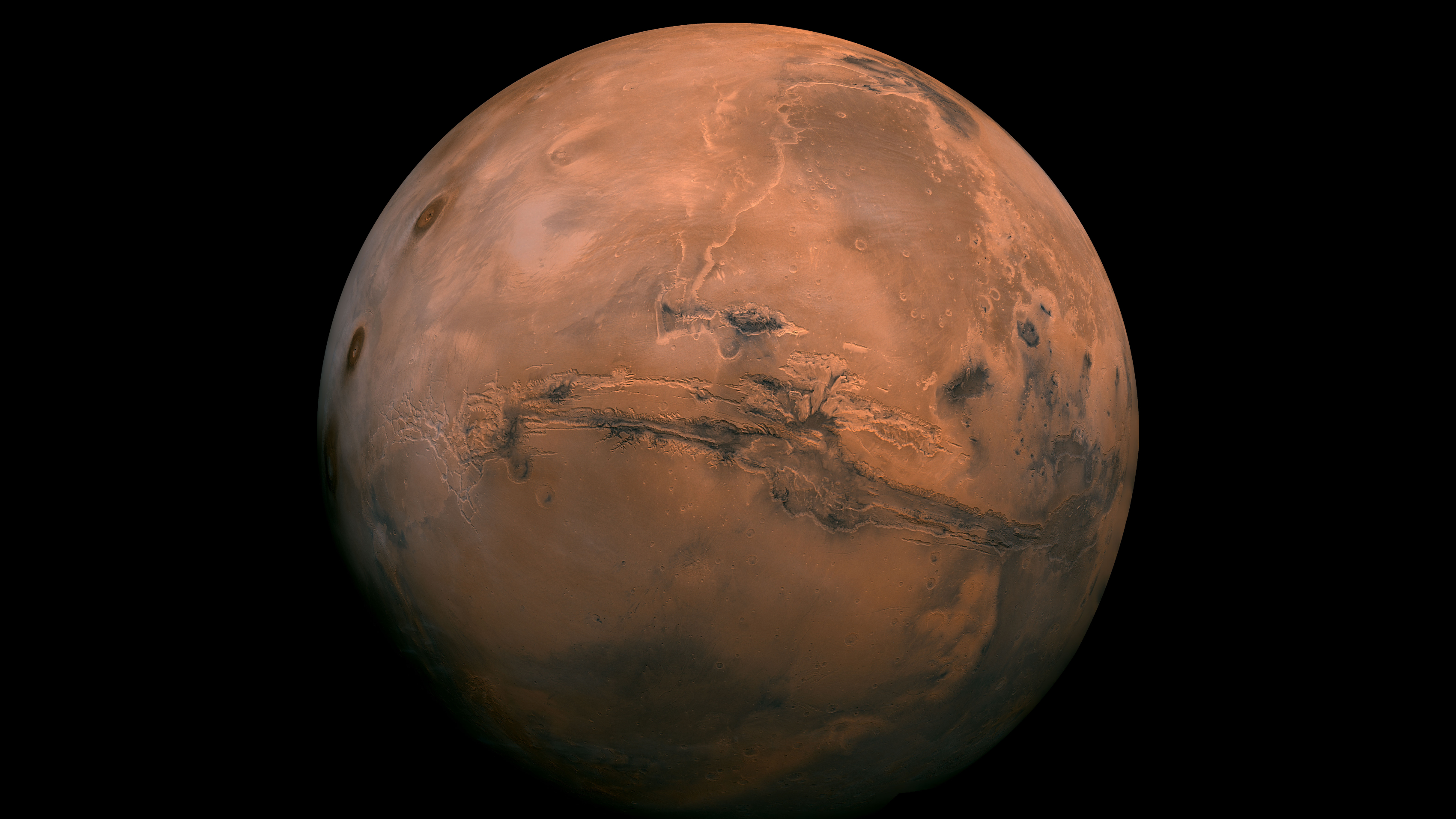
Mars is widely known for its iconic rusty red color — many people even refer to it as just the "Red Planet" — but new research suggests the Martian shade isn't just lovely to look at. The chemistry behind Mars' rosy hue may actually hold important information about our cosmic neighbor.
For decades, spacecraft and rovers have gathered data pointing to a familiar explanation behind Mars' redness: the rusting of iron minerals, namely iron oxide, in the planet's dust. That's the same compound that gives your standard "rust" on Earth its red color.
Scientists already knew that on Mars, over billions of years, iron oxide has been ground into dust and carried across the planet by powerful winds, a process still shaping the Martian landscape today. However, not all iron oxides are the same, so experts have long debated the precise nature of Martian rust. Understanding how this rust formed offers a crucial glimpse into the planet’s past environment — was it once warm and wet, or always cold and dry? And, more importantly, did it ever support life?
"We were trying to create a replica Martian dust in the laboratory using different types of iron oxide," Adomas Valantinas, a postdoctoral researcher at Brown University, formerly at the University of Bern in Switzerland where he started his work with the European Space Agency's (ESA) Trace Gas Orbiter (TGO) data, said in a statement.
To recreate the Martian dust, the new study's research team used an advanced grinding machine to refine their samples such that they matched the fine, windblown particles found on Mars. The scientists then analyzed these ground-up samples using the same techniques as spacecraft orbiting Mars would, allowing for a direct comparison with real Martian data.
"This study is the result of the complementary datasets from the fleet of international missions exploring Mars from orbit and at ground level," Colin Wilson, the TGO and Mars Express project scientist, said in the statement.
What they found was that the best match for Mars' red dust is a combination of basaltic volcanic rock and a water-rich iron oxide called ferrihydrite.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
This discovery is intriguing because ferrihydrite typically forms rapidly in the presence of cool water — meaning it must have originated when liquid water still existed on Mars' surface.
Even after billions of years of being ground into dust and scattered by Martian winds, ferrihydrite has retained its watery signature, offering a tantalizing clue about Mars' ancient past.
"The major implication is that because ferrihydrite could only have formed when water was still present on the surface, Mars rusted earlier than we previously thought," said Valantinas. "Moreover, the ferrihydrite remains stable under present-day conditions on Mars."
Data from NASA's Mars Reconnaissance Orbiter along with ground-based measurements from the Curiosity, Pathfinder and Opportunity rovers further support the identification of ferrihydrite. These observations provide crucial evidence that Mars’s red dust retains a signature of its watery past, reinforcing the idea that liquid water once played a key role in shaping the planet’s surface.
"We eagerly await the results from upcoming missions like ESA's Rosalind Franklin rover and the NASA-ESA Mars Sample Return, which will allow us to probe deeper into what makes Mars red," added Colin. "Some of the samples already collected by NASA's Perseverance rover and awaiting return to Earth include dust; once we get these precious samples into the lab, we’ll be able to measure exactly how much ferrihydrite the dust contains, and what this means for our understanding of the history of water — and the possibility for life — on Mars."
"Mars is still the Red Planet," added Valantinas. "It’s just that our understanding of why Mars is red has been transformed."
A paper about these results was published on Feb. 25 in the journal Nature.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

A chemist turned science writer, Victoria Corless completed her Ph.D. in organic synthesis at the University of Toronto and, ever the cliché, realized lab work was not something she wanted to do for the rest of her days. After dabbling in science writing and a brief stint as a medical writer, Victoria joined Wiley’s Advanced Science News where she works as an editor and writer. On the side, she freelances for various outlets, including Research2Reality and Chemistry World.
-
Unclear Engineer Because the ferrihydrite forms from exposure to cold water, I am wondering if its presence on Mars really indicates much water on the Martian surface, previously. There are apparently still annually forming ice caps on Mars. Could they temporarily form liquid water beneath the ice as they melt, allowing ferrihydrites to form, which then get blown around the planet when the ice is gone? If that sort of mechanism occurred over much of the planet's surface in the past, would it still have required lakes, rivers and oceans to produce what we see today?Reply
I do realize that there are other indications of ancient liquid surface water on Mars. So my question is more about when Mars actually turned red. Was it mainly after the water was gone, or did it happen while the planet's surface was potentially suitable for life? -
danR Reply
What I have to question is that after all the Martian landers and rovers, none of them did any simple, almost trivial, tests for the (then-presumed) hematite. And yet, almost half a century ago, two (Viking) landers undertook rather sophisticated soil tests for life itself.Unclear Engineer said:So my question is more about when Mars actually turned red. Was it mainly after the water was gone, or did it happen while the planet's surface was potentially suitable for life?
By the way, the lab simulations do not as yet falsify hematite; they are simply showing ferrihydrites to be a much more likely option. -
danR Can any of the current probes on Mars be configured to do a ferrihydrite test?Reply
"Mars Curiosity Astrobiology Rover ChemCam Instrument Has Fired 1 Million Times" —Astrobiology, Sept. 2024 -
Helio Reply
Good question, though I would assume that liquid water would need to be deeper below the surface due to the lack of air pressure.Unclear Engineer said:Because the ferrihydrite forms from exposure to cold water, I am wondering if its presence on Mars really indicates much water on the Martian surface, previously. There are apparently still annually forming ice caps on Mars. Could they temporarily form liquid water beneath the ice as they melt, allowing ferrihydrites to form, which then get blown around the planet when the ice is gone? If that sort of mechanism occurred over much of the planet's surface in the past, would it still have required lakes, rivers and oceans to produce what we see today?
From Wiki (Ferrihydrite), it's noted that this can come from hot springs and other regions, including cold water. So perhaps this article was implying that the given cold-only water was appropriate, ignoring less likely scenarios. -
Helio Reply
Yeah. Hematite does seem more likely since the chemical formula Fe2O3 is simpler than for ferrihydrite. It would seem likely that this would have been studied, and maybe it's in their formal paper.danR said:What I have to question is that after all the Martian landers and rovers, none of them did any simple, almost trivial, tests for the (then-presumed) hematite. And yet, almost half a century ago, two (Viking) landers undertook rather sophisticated soil tests for life itself.
By the way, the lab simulations do not as yet falsify hematite; they are simply showing ferrihydrites to be a much more likely option. -
Helio What impresses me is that they took the color of Mars and turned it into more solid -- I want to say "iron-clad" for some reason ;) -- evidence for liquid water.Reply
By coincidence this week, while reading"Decoding The Stores: A Biography of Angelo Secchi, Jesuit and Scientist", (Chinnici), I came across a section on the 40 exquisite color drawings of Mars by Secchi. He noted that the ice caps appeared "light yellow", but of course, they will look white in most cases.
But, in his case, Mars was low in the sky (AM2.1), which could have made a white ice cap appear more yellow than otherwise. Perhaps Rome in the late 1800s had higher than normal particle counts. Maybe oil-burning lighting at that time caused fairly high particle counts in the air, causing slight reddening of celestial light.
His best and favorite scope was a Merz 8" refractor. He placed the Sun with Capella (a yellowish star) in his three (later 5) spectral star types. Perhaps it had some chromatic issues, as well, contributing to his color account.
It's just interesting how color topics can present some fun astronomy lessons. -
johnpucci Rust on the earth requires oxygen. Does this mean that Mars had O2 sometime in the past? If so where did it come from?Reply