What color is the sun?
Spoiler alert: It's not yellow
Appearing as a whiteish yellow while overhead and redder when at the horizon, it's clear that the question "what color is the sun?" is actually more complex and difficult than it may initially sound.
The sun emits light across all the visible colors in the electromagnetic spectrum fairly evenly. When these come together united in sunlight the sun appears white. This is useful because if this balance was thrown off colors less favored would be difficult to see.
The sun is officially classified as a yellow dwarf star or a G2 V star. G2 designates the second hottest stars of the yellow G class of stars with surface temperatures between about 5,300 Kelvins (K) and 6,000 K. The V in this label represents a hydrogen-burning main sequence star or dwarf star. In terms of mass, the sun is at the upper end of this classification of stars.
Related: How to observe the sun safely (and what to look for)

Robert Lea is a science journalist based in the U.K. whose work has been published in Physics World, New Scientist, Astronomy Magazine, All About Space, Newsweek and ZME Science. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University.
When astronomers observe the sun they do so across a range of wavelengths of light — or electromagnetic radiation — including visible light and light not visible to the naked eye.
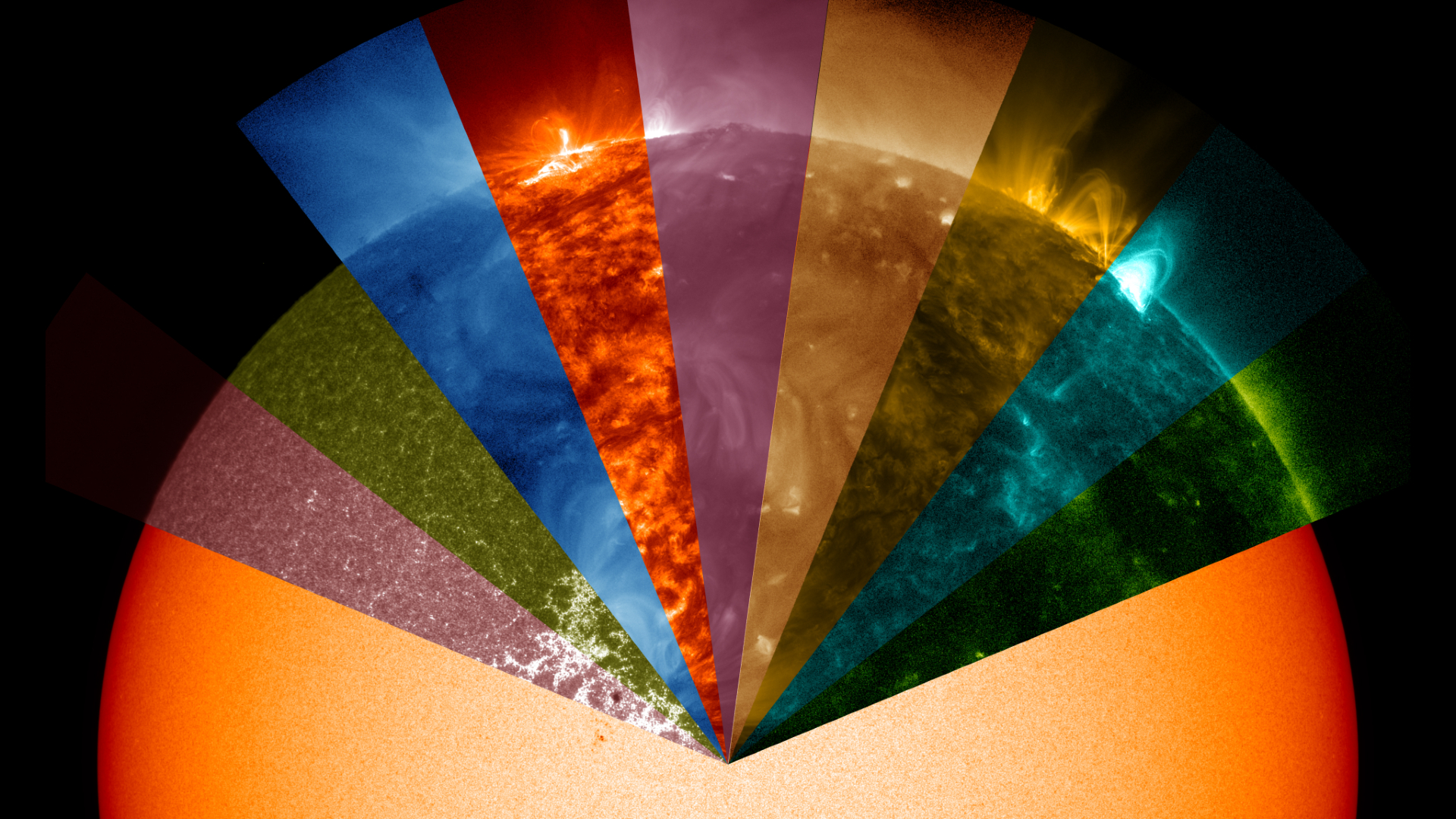
These images of the sun in non-visible light are often reproduced in visible colors not usually associated with the sun. That means that is a veritable rainbow of exotically colored images of our star online that represent observations in different wavelengths of light.
Understanding the color of the sun is tied to our understanding of the electromagnetic spectrum and these different wavelengths.
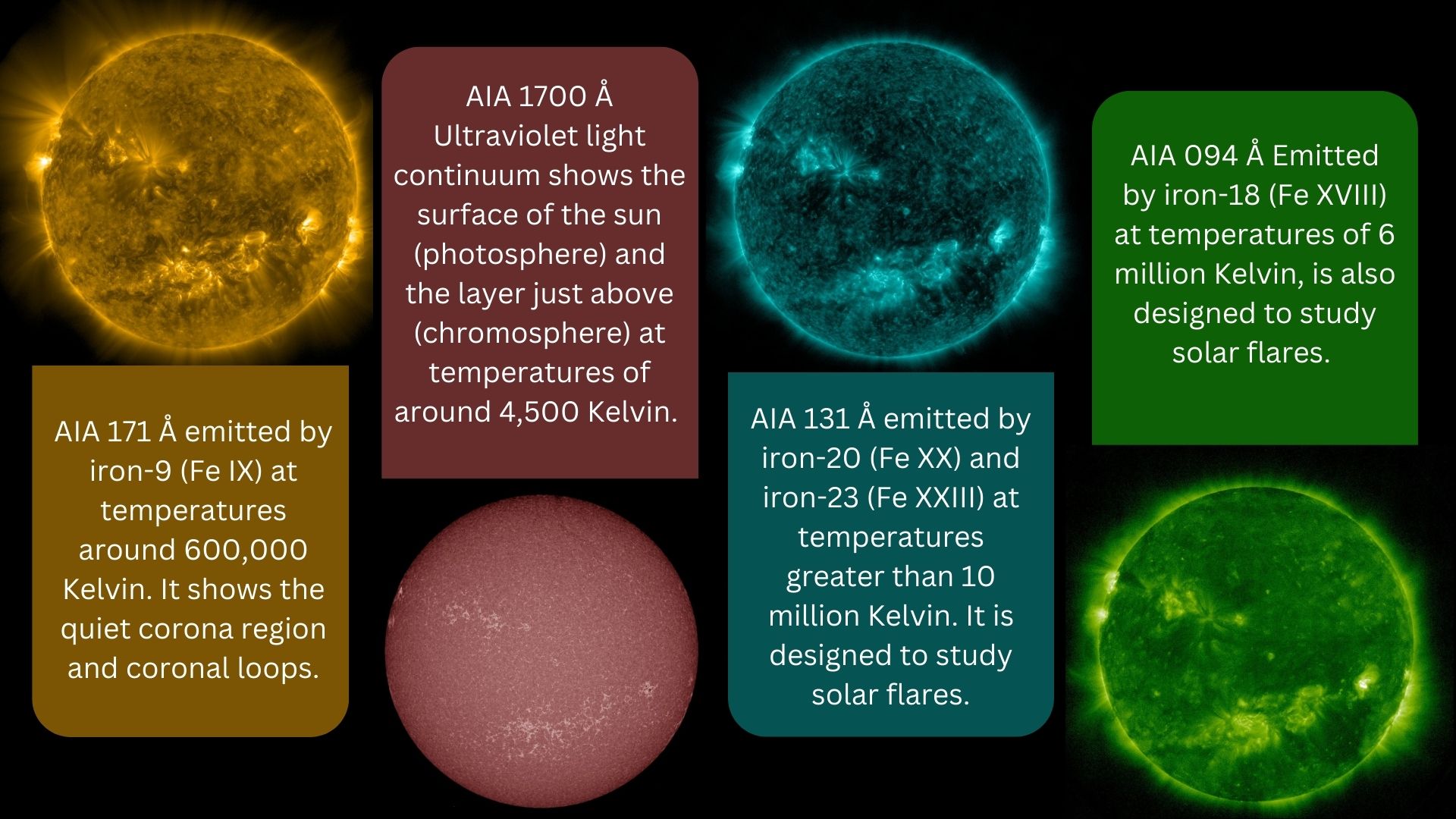
Specialized instruments on NASA's Solar Dynamics Observatory (SDO) capture images in 10 different wavelengths, measured in angstroms (Å). Here are some examples of four images captured at 171 Å, 1700 Å, 131 Å and 094 Å by the SDO's Atmospheric Imaging Assembly (AIA) instrument.
What is the electromagnetic spectrum?
The visible light spectrum is just a tiny fraction of the electromagnetic spectrum that ranges from radio waves to microwaves and infrared light to X-rays and gamma-rays.
These different types of electromagnetic radiation are comprised of photons with the key difference between them being their wavelength, frequency, and the energy these photons carry.
These three properties are intrinsically related. Wavelength and frequency come from the wave-like properties of light with wavelength defined as the distance between the peaks of a wave and frequency defined as the number of wave peaks that pass a set point per second.
Wavelength and frequency are inversely proportional, so a longer wavelength means a reduction in the number of waves that pass a set point per second thus a reduction in frequency. Conversely, shortening the wavelength means more waves pass a set point per second and thus an increase in frequency.
Wavelength and frequency are linked to energy by a factor called Planck's constant and as a result, energy is proportional to frequency and inversely proportional to wavelength.
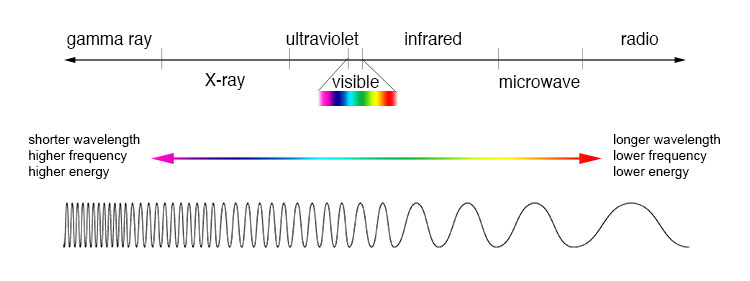
Applying this to the electromagnetic spectrum, radio waves have a long wavelength and thus a low frequency and little energy. On the other end of this spectrum, gamma rays have extremely short wavelengths and thus high frequencies and carry a lot of energy.
The sun emits electromagnetic radiation across this spectrum except for gamma rays which it does generate in its core, but these high-energy photons bounce around the dense interior of the sun losing energy as they do so.
This means when they finally escape the sun it is as longer wavelength, lower frequency, and lower energy photons.
The quirks of the electromagnetic spectrum, energy, wavelengths, and frequency, have a discernable effect on how we see the sun from Earth.
Why does the sun look red sometimes?

Most of us have, at some time, looked at the setting or rising sun on the horizon and noticed that it has taken on a red hue not usually seen when it is overhead. Likewise, as children we probably all asked a teacher or parent why the sky is blue.
It turns out, both phenomena are related and are connected to the fact the sun emits photons across the visible light spectrum and these photons have different qualities.
Blue light has a shorter wavelength than red or yellow light and shorter wavelength light is more easily scattered by gas molecules — a process called Rayleigh scattering. That means as white light from the sun travels through Earth's atmosphere blue photons get scattered more by molecules in the atmosphere while red photons slip right past them with little scattering.
As a result, blue light has bounced all around the sky before it hits your eye. Photons of violet light are also scattered like this, but the sky isn't violet to us because the sun produces more blue photons and the human eye is more receptive to blue than it is too violet.
Related: Why is the sky blue?
Photons of red, orange and yellow photons reach your eye pretty much directly with little scattering when the sun is overhead, say at midday, and its light doesn't have to travel through much of Earth's atmosphere to reach the planet's surface.
That's why the sky looks blue and the sun white with a yellow, red or orange tinge.
When the sun is on the horizon, at sunset, however, the situation is different as its light has to travel further and through a denser region of Earth's atmosphere to reach the planet's surface.
This results in blue light being more strongly scattered and thus mostly removed from white light from the sun, this leaves mostly red light and makes the sun appear red.
For this same reason, dust particles and pollution in the air can also cause the sun to take on dull shades of red and yellow or can even cause grey skies.
Of course, the changes in the color of the sun as a result of these factors are superficial. The sun just appears to have taken on a different color. Eventually, however, our star will actually physically change color.
This will be more than a mere palette swap and will be disastrous for the Earth and the planets in the inner solar system.
Will the sun ever change color?

In around 5 to 6 billion years the sun will exhaust the hydrogen that it converts to helium at its core as it comes to the end of its main sequence lifetime.
The end of this nuclear fusion process also signals the end of outward pressure provided the energy it generates. Thought-out the sun's lifetime this outward radiation pressure has protected the sun from collapsing under its own inward gravitational pressure.
When this 10 billion year or so balancing act ends the core of the sun will begin to collapse creating enough pressure to fuse helium in the core into heavier elements like carbon, nitrogen, and oxygen. The heat from the core will cause layers of the sun on top of the core to 'puff out' and hydrogen will begin fusing into helium in these layers.
As the outer material of the sun expands outwards the temperature of these layers will drop and this will cause the energy of photons it emits to drop too. Lower energy light, as seen above, moves towards the red end of the electromagnetic spectrum.
This means the expanding sun will take on a reddish brown hue as it enters the aptly named red giant phase of its existence. Just as red often indicates 'danger' in nature, this expansion and reddening of the sun represent the final risk for the Earth.
Many stars in the period of their existence can swell to as much as 400 times their original diameter. The outer shell of the sun is predicted to expand out to around 100 times the star's main sequence diameter. This means it will touch the orbit of Mars during this red giant phase.
As a result the inner rocky planets of the solar system, Mercury, Venus, Earth, and Mars, will be swallowed by the expanding reddening star and destroyed. This won't be the final color change for the sun.
As the outer shell of stellar material spreads out and cools further it leaves behind what was once the core of the sun. As this stellar ember continues to cool, after about 10 billion years it will become a smoldering white dwarf with a whitish-blue coloration.
When what was once the sun's core runs out of elements to fuse it will fade and scientists think after trillions of years the sun become a black dwarf — a completely dead inert husk emitting no light what-so-ever and lunging what remains of the solar system into darkness.
Additional information
The Parker Solar Probe is the first craft from Earth explicitly designed to 'touch the sun' and will travel as close as 4 million miles to its surface revolutionizing our understanding of our star. Read about its science objectives with NASA. NASA's heliophysics missions study the sun in great detail and investigate its effects on interplanetary space, learn more about these missions with NASA's mission fleet page. Take an in-depth tour of the sun courtesy of ESA's Solar Orbiter, zoom in and explore a high-resolution image of the sun and learn how the orbiter measures the temperature of the different layers of the sun's atmosphere.
Bibliography
Introduction to the Electromagnetic Spectrum. NASA. Accessed Oct. 18, 2022, from: https://science.nasa.gov/ems/01_intro
The Electromagnetic Spectrum, Imagine the Universe: NASA, Accessed Oct. 18, 2022, from: https://imagine.gsfc.nasa.gov/science/toolbox/emspectrum1.html
Chapter 6: Aging Into Gianthood, NASA Exoplanet Exploration, Accessed Oct. 18 from: https://exoplanets.nasa.gov/life-and-death/chapter-6/
Light, Britannica, Accessed Oct. 18, 2022 from: https://www.britannica.com/science/light
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Robert Lea is a science journalist in the U.K. whose articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space, Newsweek and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University. Follow him on Twitter @sciencef1rst.